Challenges Facing Vaccine Logistics and Temperature Monitoring
When handling extremely sensitive goods such as vaccines, the list of requirements and logistical challenges grows exponentially.
Learn more

Vaccine Logistics and Temperature Monitoring
Everything from quality control to inventory management to varying infrastructure and cold chain have to be carefully managed every step of the way to avoid wastage, regulatory penalties and financial loss. What’s more, vaccine temperature monitoring is the responsibility of each stakeholder in the supply chain – manufacturer, importer, wholesale, distribution center, hospital or clinic – which means every hand-off carries significant risks in compromising the product. This is why having a purpose-built solution for vaccine cold chain monitoring is critical.
Ensuring cold chain for vaccine logistics and temperature monitoring
Vaccines have special needs compared to other pharmaceutical products. There are a variety of factors that impact and pose challenges to the vaccine supply chain:
Maintaining cryogenic conditions
Many vaccines require deep refrigeration or even freezing to remain effective. Any break in the cold chain can lead to vaccines becoming ineffective, which can result in wasted doses or reduced efficacy. To maintain the cold chain, vaccines need to be stored and transported in specialized containers and cryogenic equipment from their manufacturing facility to the end-user. Cryogenic storage and shipping may also require specialized logistics and transportation arrangements. Proper coordination, planning, and communication among all stakeholders involved in the transportation process are essential.
Specialized temperature monitoring
Ensuring that vaccines remain at the right temperature during storage and transportation is crucial. However, temperature monitoring can be a challenge in areas with poor or limited infrastructure, where it may be difficult to install and maintain temperature monitoring equipment. Also, extreme cold temperatures can negatively affect the performance of electronic components and circuits, causing them to malfunction or fail. This is why, when handling vaccine shipping and storage, temperature data loggers need to be specially built to withstand deep-freeze conditions and extreme temperature differences.
Complex data management
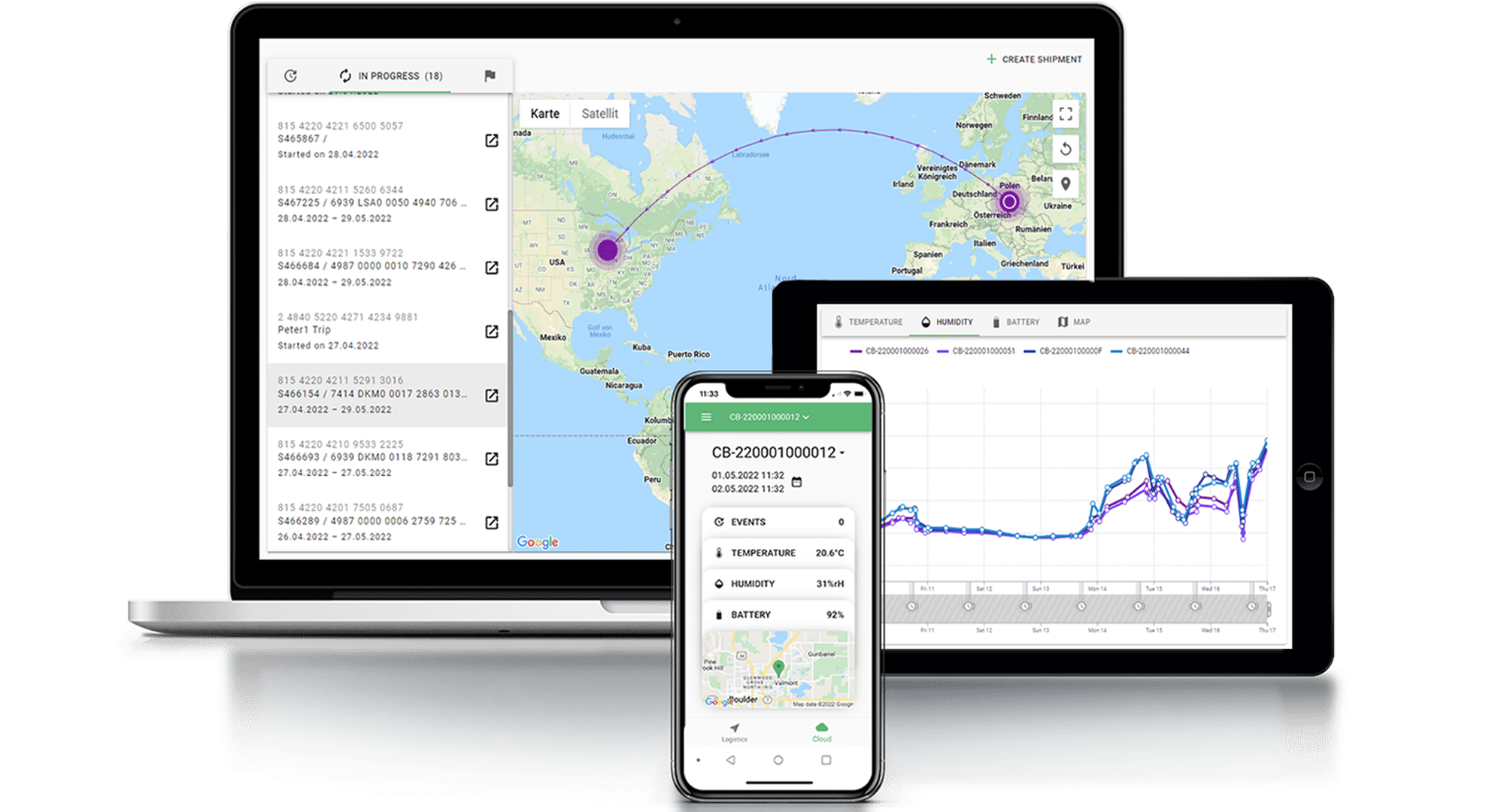
Tracking inventory levels, monitoring temperatures, and managing distribution schedules is a big challenge for the logistics managers. Effective data management is crucial for ensuring that vaccines are distributed and administered in a timely and efficient manner. To help manage this data, some have implemented vaccine information management systems (VIMS) or electronic vaccine registries (EVRs) that can track vaccine doses and monitor vaccine inventory. Smart devices that record temperature, humidity, shock, GPS location and light are also in use to ensure proper data records and vaccine safety.
Varying seasonal demand
Seasonal vaccines (i.e. influenza) present unique challenges for supply chain logistics and temperature monitoring. Depending on the severity of the flu season, demand for influenza vaccines may vary widely from year to year. This can make it difficult for vaccine manufacturers to predict demand and plan production accordingly. Specialized data loggers help provide valuable data on the performance of the cold chain during seasonal demand. This data can be analyzed to identify patterns and trends, helping manufacturers, distributors, and healthcare providers better understand the requirements and challenges of specific vaccine seasons.
Outbreaks and pandemics
Pandemics, such as COVID-19, can place significant strain on vaccine supply chains. In addition to the challenges associated with manufacturing and distributing large quantities of vaccines quickly, there may be increased demand for certain types of vaccines or a shortage of critical supplies, such as cold storage units. Vaccine supply chains often face capacity constraints during pandemics, with limited infrastructure, resources, and personnel available to handle the increased demand. The use of data loggers helps logistics personnel and healthcare providers to quickly identify and address logistical or temperature issues. Prompt decision-making helps minimize the risk of vaccine wastage or reduced efficacy, ensuring that an adequate supply of high-quality vaccines is available during periods of increased demand.
The Tec4med Advantage: Vaccine Logistics and Temperature Monitoring System
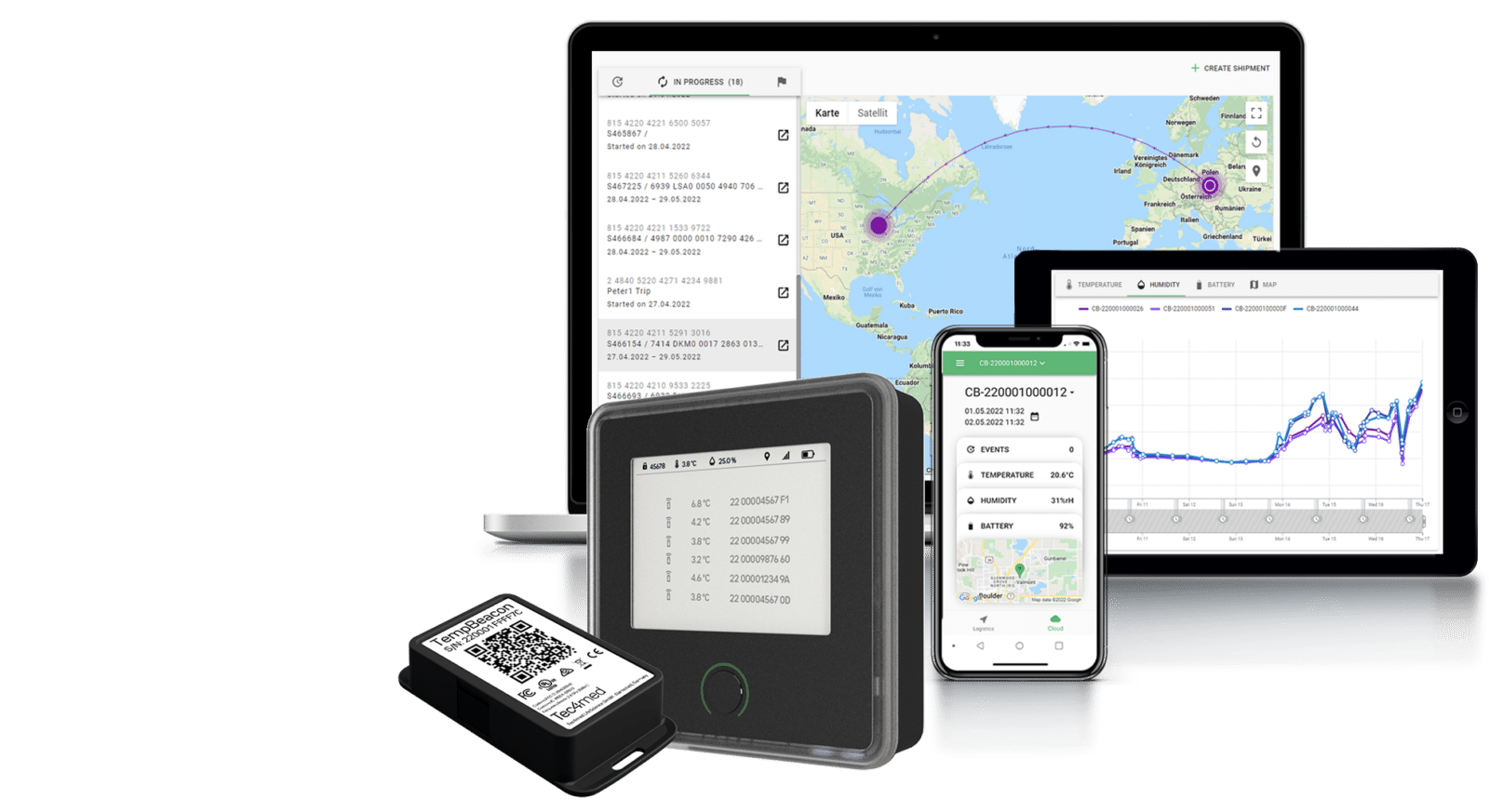
Tec4med offers a comprehensive smart solution to effectively address the challenges with vaccine logistics and ultra-low temperature monitoring. It combines reusable cryogenic packaging, such as insulated containers and dry ice shippers, as well as specialized sensors and devices for data recording in cryogenic conditions. These devices track temperatures at various points along the vaccine supply chain and pull all sensor data automatically into the web-based cloud server, ensuring proper audit trail. Tec4med solution provides full supply chain visibility and eliminates the need for manual evaluation, saving billable time and potential errors or inaccuracies.
Compliant
with pharma GDP, GMP, GAMP5, ISO17025, FDA 21 CFR Part 11
Fully IATA-compliant
and can be used on aircraft with no further exceptions
Worldwide
data recording with easy in-app analyses of all sensor data
Risk and cost
reduction through more efficient and safe work processes
