Vaccine Cold Chain Monitoring
Proper handling and optimal supply chain visibility are critical to ensuring the efficacy and safety of vaccine transportation and storage. With every hand-off within the supply chain, vaccines are exposed to potential risk and proving that the product shipment wasn’t compromised is critical.
Free Consultation

Uninterrupted temperature monitoring every step of the way
Proper handling and optimal supply chain visibility are critical to ensuring the efficacy and safety of vaccines. With every hand-off within the supply chain, vaccines are exposed to potential risk and proving that the product shipment wasn’t compromised is critical.
To help manufacturers and logistics personnel better ensure that the vaccines are viable and delivered in optimal conditions, they are required to use cold chain monitoring and data logging to comply with Federal regulations, starting from the manufacturing process through to when they are administered to individuals.
The World Health Organization (WHO) also provided strict guidelines and regulations for the handling, storage, and transportation of vaccines that are mandatory for all cold chain partners to comply with.
Key WHO regulations include
- Temperature control: Recommended temperature range depends on the type of vaccine.
- Packaging and labeling: Identification and traceability of vaccine shipments
- Transport equipment: Suitability for the type and volume of vaccines being transported
- Monitoring and record-keeping: Recording and storing of ambient data at regular intervals
- Quality control: Inspection upon receipt
- Security: Preventing theft and tampering
Vaccine Cold Chain Monitoring
Vaccines are required to have constant monitoring through data logging, as you must be able to demonstrate compliance with regulatory standards through documentation and reporting if/when government audits occur.
Several technologies and methods are used for vaccine cold chain shipment monitoring, including temperature loggers, RFID tags, and GPS tracking. But many traditional cold chain solutions are limited in their functionality and require a lot of manual input. These limitations can significantly reduce the security, accuracy and ability to detect undesired environmental changes.
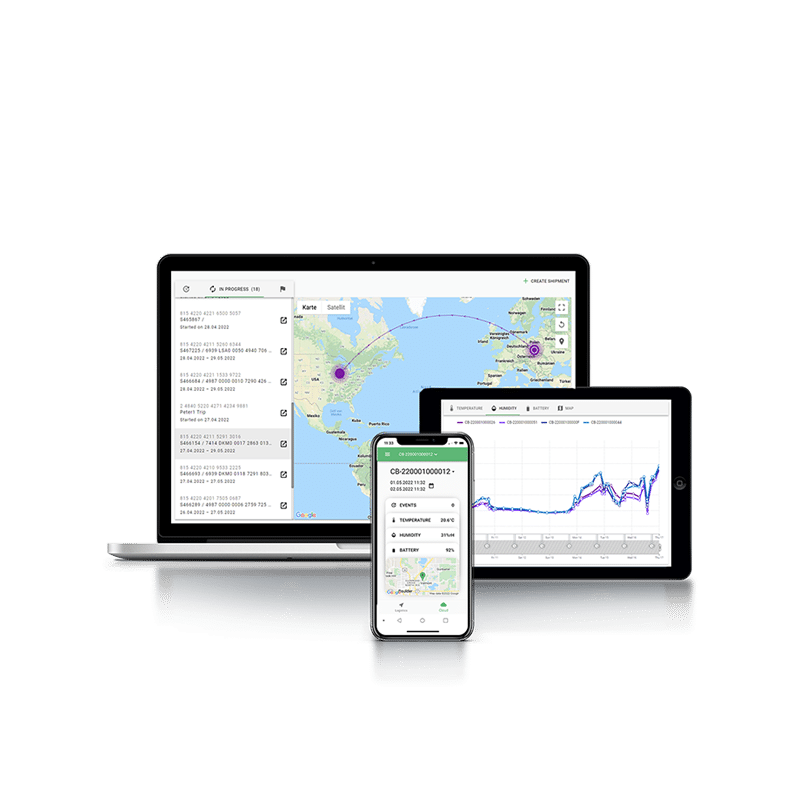
More advanced solutions, like Tec4med’s SmartTag data logging device and Tec4Cloud intelligence software, deliver higher accuracy, real-time monitoring of all parameters (e.g. temperature, humidity, impact) and require minimal to no manual involvement.


Challenges Facing Vaccine Monitoring
Everything from quality control to inventory management to varying infrastructure and cold chain have to be carefully managed every step of the way to avoid wastage, regulatory penalties and financial loss. What’s more, vaccine temperature monitoring is the responsibility of each stakeholder in the supply chain – manufacturer, importer, wholesale, distribution center, hospital or clinic – which means every hand-off carries significant risks in compromising the product. This is why having a purpose-built solution for vaccine cold chain monitoring is critical.
Regulatory Compliance for Vaccine Distribution
Vaccine storage and handling standards pose high expectations and demands on the supply chain logistics. Maintaining optimal ambient temperatures is critical for the safety and efficacy of vaccines; it requires compliance with highly technical conditions and regulations. Vaccine manufacturers, distributors, logistics personnel and healthcare providers must follow all applicable regulations and guidelines to ensure the appropriate storage and temperature monitoring.


Clinical Trials Simplified With Always-on Vaccine Monitoring
Clinical trials play an essential role in the overall vaccine development process and have inherently complex storage and distribution requirements. Time and temperature-sensitive, vaccine clinical trials involve deliveries to multiple sites in different geographical locations and require around-the-clock ambient monitoring that needs to meet local regulatory guidelines.
Benefits of Real-time Vaccine Monitoring
As a pharmaceutical manufacturer, quality assurance manager or logistics staff, you require precise environmental monitoring that you can prove. The latest advancements in real-time monitoring for vaccine distribution is transforming the way vaccines are handled, stored, and delivered.

Key Benefits

Key Benefits
By providing exportable, detailed temperature logs and alerts through Tec4Cloud, SmartTag devices help demonstrate compliance with regulatory standards and improve audit readiness. You will gain real-time visibility into the status of vaccines as they move through the cold chain, enabling you to track shipments and respond quickly if any issues arise.
Compliant with pharma
GDP, GMP, GAMP5, ISO17025, FDA 21 CFR Part 11
Fully IATA-compliant
and can be used on aircrafts with no further exceptions
Worldwide
data recording with easy in-app analyses of all sensor data
Risk & cost
reduction through more efficient and safe work processes
