Cold Chain Challenges with Cryogenic Storage and Shipping
Ensuring specialized monitoring for ultra-low temperatures
Pharmaceuticals and bio samples are often deep-frozen, or stored at cryogenic temperatures, for preservation of efficacy, stability, shelf-life extension, specialized application, and other reasons. Cryogenic shipping and storing of pharmaceuticals is a critical process that requires careful handling to maintain the integrity and efficacy of temperature-sensitive medications, samples and vaccines. Because of its extreme temperatures, cryogenic distribution presents higher risks, stricter regulatory requirements and more complex logistics.Free Consultation

The main concerns for those managing cryogenic storage and shipping are:
Maintaining ultra-low temperatures: Specialized storage containers, such as liquid nitrogen dewars or dry ice shippers, are needed to maintain temperatures of below -196°C for liquid nitrogen and below -78°C for dry ice during transportation and storage. It also requires the use of specialized monitoring devices, like CryoBeacon, capable of reading and storing cryogenic temperatures.
Packaging requirements: Packaging materials and methods need to undergo proper testing to verify that they can withstand ultra-low temperatures without cracking, leaking or becoming brittle. Specialized cryogenic packagings, such as insulated containers or dry ice shippers, may be needed to provide the necessary thermal protection during transportation.
Handling hazards: If mishandled, direct contact with cryogenic materials (i.e. liquid nitrogen, dry ice) can cause severe cold burns and frostbite. Liquid nitrogen can also release oxygen-depleting gasses as it evaporates, exposing staff to risks in poorly ventilated spaces.
Regulatory compliance: Cryogenic transportation of pharmaceuticals is subject to a series of strict regulations, guidelines, and standards set by the FDA (Food and Drug Administration) and IATA (International Air Transport Association). Compliance with these regulations includes proper documentation, labeling, and handling procedures.
Complex logistics and transportation: Cryogenic storage and shipping may require specialized logistics and transportation arrangements. Proper coordination, planning, and communication among all stakeholders involved in the transportation process are essential.
Higher costs: The cost of cryogenic storage and shipping is usually higher due to safety risks, required specialized equipment and handling restrictions. Additionally, compliance with regulations requires specialized training, documentation, and quality control measures, which add to the running costs.

Key Benefits: Tec4med Cryogenic Storage and Shipping
Tec4med offers a comprehensive solution to effectively address the challenges with cryogenic distribution. It combines reusable cryo containers and shippers, sensors and devices for data recording, as well as Cloud-based software for monitoring and maintaining temperature ranges. This trifecta provides full supply chain visibility and eliminates the need for manual evaluation, saving billable time and potential errors or inaccuracies.
Beacons & Gateway
Beacons & Gateway
Cryogenic Data Tracking
Tracking
temperature (-200° to +80°C / -328°F to +176°F)
Automated
data upload to the cloud
Calibrated
for GxP conformity
Tec4Cloud
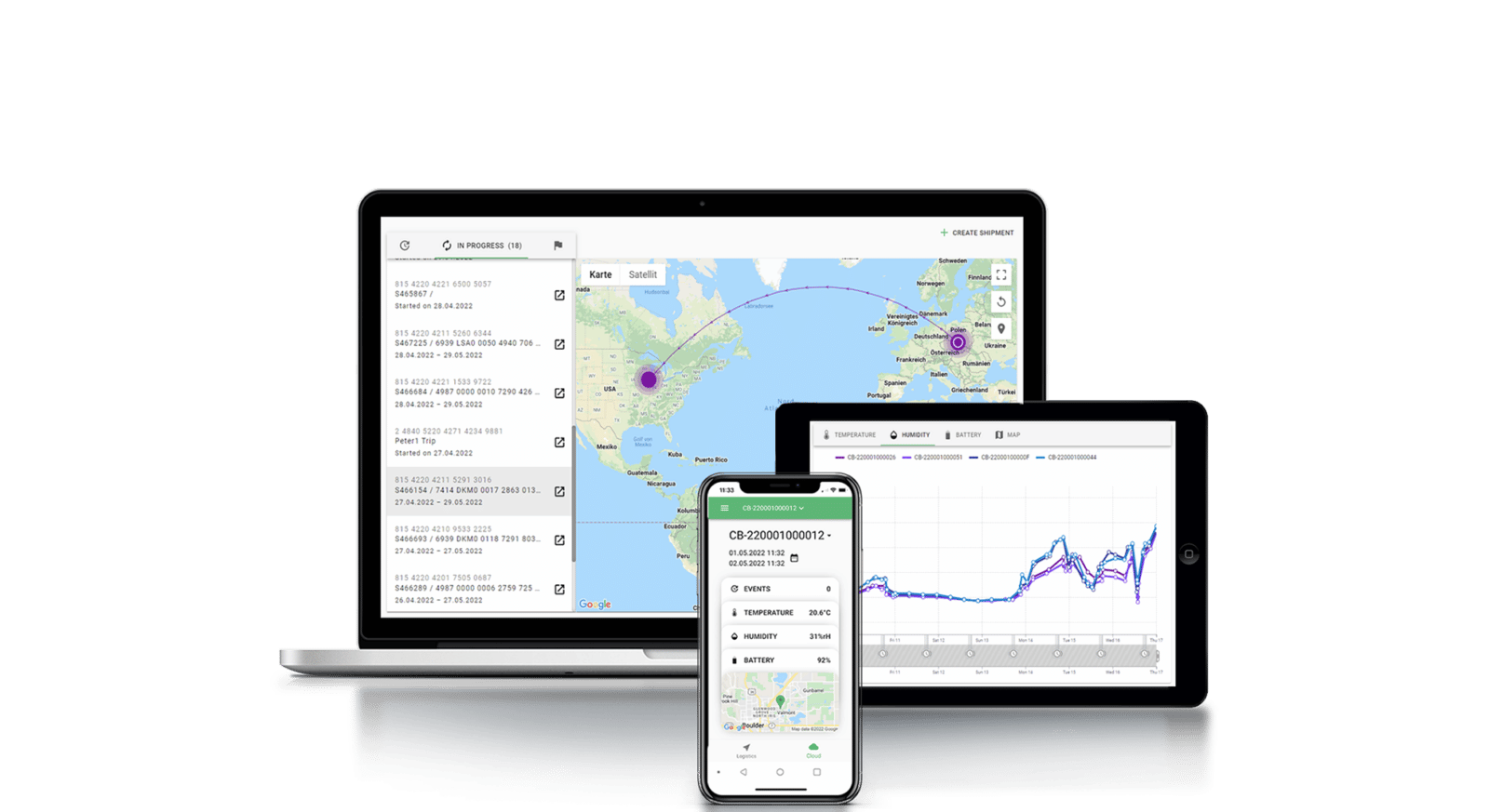
Tec4Cloud
Cryogenic Data Monitoring
Compliant
with pharma GDP, GMP, GAMP5, ISO17025, FDA 21 CFR Part 11
Worldwide
data recording with easy in-app analyses of all sensor data
Risk and cost
reduction through more efficient and safe work processes
