Dry Ice and Liquid Nitrogen (LN2) Requirements for Cryo Storage and Shipping
Both dry ice and liquid nitrogen (LN2) are commonly used as refrigerants in the cryogenic distribution of pharmaceuticals and other temperature-sensitive products. The choice between using dry ice and LN2 depends on several factors, including the specific product being shipped, the desired temperature range, and the duration of the shipment. There are various safety hazards associated with transporting or storing cryogenics due to the inherent nature of the gasses involved (carbon dioxide and nitrogen) and their extreme cold conditions.
Free Consultation
Mitigate safety hazards when storing or transporting cryogenics.
There are two important factors to consider when handling cryogenic shipments and storage.
- Safety: The safe handling of cryogenic materials is critical and needs to undergo strict regulatory requirements. If not handled properly, dry ice and LN2 can cause health hazards, such as frostbite and asphyxiation.
- Specialized equipment: Electronic components generally don’t do well in cold conditions. Ensuring that your monitoring devices, such as data loggers, function properly when dealing with cryogenic shipments is crucial.


Temperature range
- Dry ice can maintain temperatures between -60°C and -80°C. Its average sublimation temperature is -78°C, however, based on various factors, such as vibration, air pressure, pollution of the dry ice, temperatures can go much lower.
- LN2 can maintain temperatures as low as -196°C. If the product needs to be maintained at extremely low temperatures, LN2 may be a better choice.
Duration of shipment
- Dry ice has a shorter lifespan compared to LN2 and typically lasts between 24-48 hours
- LN2 can last up to several weeks. If the shipment requires a longer transit time, LN2 may be a better choice.
Safety considerations
- Dry ice is generally considered to be less hazardous and easier to handle, making it a more convenient choice for some applications.
- Handling and transporting LN2 requires specialized training and equipment due to the risks associated with its extremely low temperatures.
What to consider when using real-time monitoring devices for dry ice or LN2
Extreme cold temperatures can negatively affect the performance of electronic components and circuits by causing them to malfunction, operate in reduced performance, or leading to damage or failure. This is why, when handling cryo shipping and storage, data loggers need to be able to withstand deep-freeze conditions and extreme temperature differences.
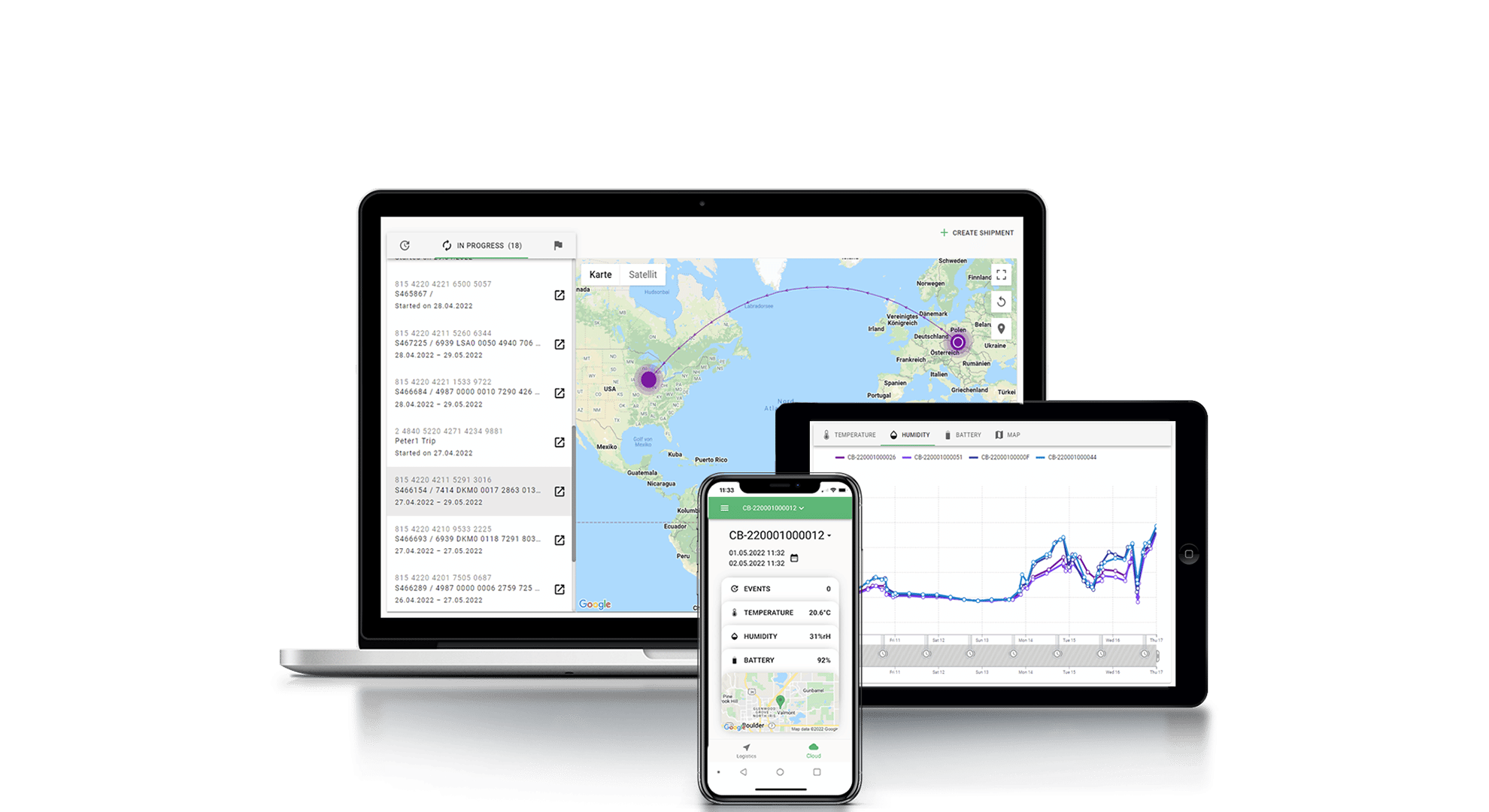
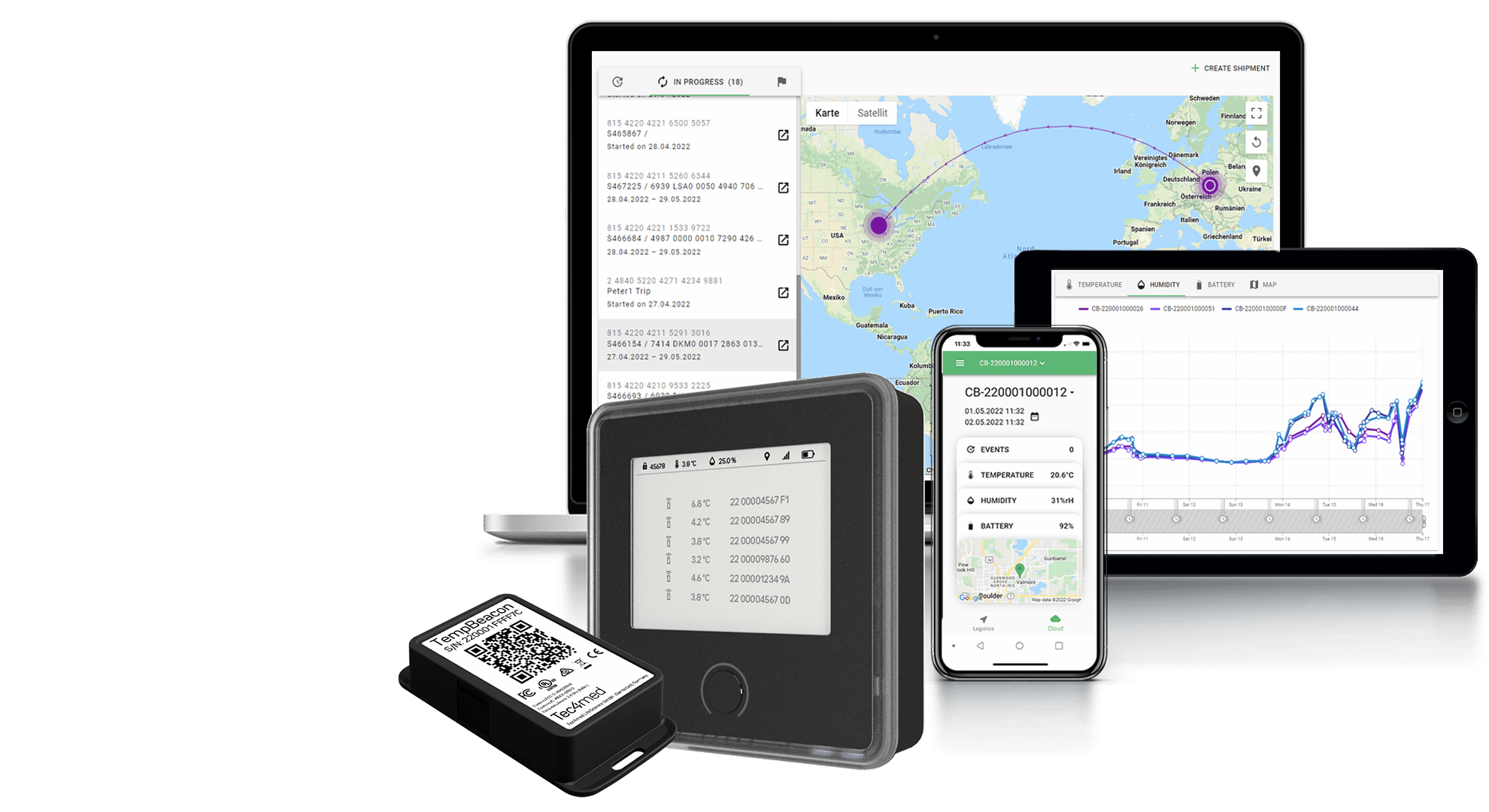
Electronic devices designed for use in extreme temperatures often incorporate special features such as insulated batteries, thermal management systems, and moisture-resistant coatings. For example, Tec4med’s purpose-built wireless data loggers can withstand temperatures of -200°C, 100% rH humidity, and are pre-calibrated according to ISO17025 secure high standards.
Regulatory compliance and requirements
The US Food and Drug Administration (FDA), the Occupational Safety and Health Administration (OSHA), and the Department of Transportation (DOT) set the regulatory requirements for handling cryogenic distribution. Regulations for the use of dry ice and LN2 vary depending on the specific application and product being transported.
By correctly selecting data loggers and monitoring devices, you can ensure that your cryogenic storage and shipment processes are properly monitored and meet regulatory requirements.
Dry Ice
- According to the DOT regulations, packages containing dry ice must be labeled with the "Dry Ice" or "Carbon Dioxide, Solid" label.
- The amount of dry ice that can be transported is limited (typically 5.5 pounds per package or 440 pounds per vehicle).
- Packages containing dry ice must be adequately ventilated to prevent the buildup of carbon dioxide gas.
- All packages containing dry ice must be properly marked and labeled to ensure proper handling during transport.
Liquid Nitrogen
- The transportation of LN2 requires the use of specialized equipment, including cryogenic storage tanks, cryogenic containers, and vacuum-insulated pipes.
- Liquid Nitrogen tanks must be transported in a vented container to prevent pressure buildup and to ensure that no dangerous volume accumulates in the ambient air.
- Personnel involved in the transportation of LN2 must be properly trained on the safe handling of cryogenic materials.
- All containers holding LN2 must be properly labeled with the "Cryogenic Liquid" label and the product name.
The Tec4med Advantage
Tec4med’s Cryogenic Data Tracking & Monitoring solutions offer a comprehensive way to handle distribution involving both dry ice and LN2, including reusable cryo containers and shippers, sensors and devices for data recording, as well as Cloud-based software for monitoring and maintaining temperature ranges. They effectively provide full supply chain visibility and automate evaluation processes, preventing manual errors or inaccuracies for optimal audit trail and compliance management.

Key Benefits
Key Benefits
Compliant with
pharma GDP, GMP, GAMP5, ISO17025, FDA 21 CFR Part 11
Fully IATA-compliant
and can be used on aircraft with no further exceptions
Worldwide
data recording with easy in-app analyses of all sensor data
Risk and cost
reduction through more efficient and safe work processes
